|
一、The structure of a protein encodes fluorescence. Such fluorescent proteins include green fluorescent protein (GFP), yellow fluorescent protein (YFP), red fluorescent protein (RFP), and various derivatives thereof.
1.The Ancestor of Fluorescent Proteins——Green Fluorescent Protein (GFP)
Green Fluorescent Protein (GFP): Based on the crystal structure of GFP, site-directed or random mutagenesis is used to identify GFP mutants with enhanced functions. The most widely used ones include Enhanced Green Fluorescent Protein (EGFP) and Emerald (a GFP variant emitting emerald-green fluorescence), as well as various other derivative GFPs such as destabilized Enhanced Green Fluorescent Protein (dEGFP), Enhanced Yellow Fluorescent Protein (EYFP), Enhanced Blue Fluorescent Protein (EBFP), and Enhanced Cyan Fluorescent Protein (ECFP). These derivative GFPs emit fluorescent light of different colors.
.png)
2.Red Fluorescent Protein (RFP)
Although Green Fluorescent Protein (GFP) has a wide range of applications, its emission spectrum is limited to 440–529 nm. This results in relatively high background during intracellular imaging, making it unable to achieve deeper fluorescent labeling in the subcutaneous tissue of living organisms. In contrast, Red Fluorescent Protein (RFP) has longer excitation and emission wavelengths, leading to lower background in intracellular imaging. Additionally, RFP can be used for co-labeling with GFP.
The first red fluorescent protein (RFP) used in research is DsRed (with an emission peak at 583 nm), which is derived from corals and exhibits strong fluorescence and good stability. However, DsRed tends to form oligomers and has a slow maturation rate. Mutants of DsRed include mBanana, mOrange, tdTomato, mTangerine, mStrawberry, and mCherry. Among these, mStrawberry and mCherry have emission peaks at 596 nm and 610 nm, respectively, with brightness levels of approximately 75% and 50% that of Enhanced Green Fluorescent Protein (EGFP).
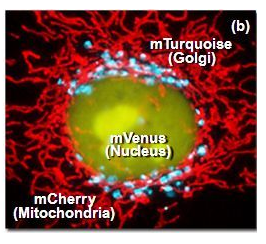
3.Physical Properties of Commonly Used Fluorescent Proteins
.png)
二、Derived from phycobiliproteins found in algae and plants
These proteins use phycobiliprotein cofactors to absorb light energy, including phycoerythrin (PE), allophycocyanin (APC), and peridinin-chlorophyll (PerCP).
Features:
It has a large Stokes shift (75–200 nm) and a stable emission spectrum.
Due to the large size of phycobiliproteins, they typically have a 1:1 ratio of protein to fluorescent dye during the conjugation process, making them highly useful for quantitative flow cytometry. However, they are susceptible to photobleaching, so prolonged or repeated exposure to excitation light sources is not recommended.

|