|
一、Introduction
Since the invention of the optical microscope, researchers have been striving to enhance the detectability of target signals relative to background noise. Fluorophores have emerged as mainstream signal-generating labels due to their brightness, well-defined excitation and emission spectra, reproducibility, and ease of use in biological systems. However, the use of fluorophores also introduces certain limitations. Owing to the fundamental physical properties of photons, features below the diffraction limit cannot be resolved. Furthermore, individual fluorophores—even clustered ones—are difficult to distinguish from the background using conventional fluorescence microscopes. Therefore, diffraction and background signals are key considerations in single-molecule fluorescence detection.
.png)
Various methods that successfully overcome the diffraction limit of light are collectively referred to as super-resolution microscopy. Over the past few decades, super-resolution microscopy has undergone profound improvements, driven by transformative advances in instrumentation, optics, fluorophore chemistry (including autofluorescent dyes), and post-imaging computational processing.
The improvement of fluorophore signal-to-noise ratio (SNR) mainly relies on advanced imaging setups, such as total internal reflection fluorescence (TIRF) microscopy—where the evanescent wave generated excites fluorophores only near the interface—or confocal microscopy, which uses a pinhole to block out-of-focus light from reaching the detector. While research laboratories previously had to manually construct single-molecule detection systems, an increasing number of commercial turnkey solutions have become available over the past few decades.
Despite these advances, many research laboratories still lack access to specialized single-molecule equipment. Fluorescence microscopy remains a primary tool in cell biology and immunofluorescence imaging. Since conventional fluorescence microscopy is not optimized for signal resolution, distinguishing individual molecules requires the use of bright fluorescent conjugates.
二、Experimental Results
1.Fluorescence Measurement on Glass Surface
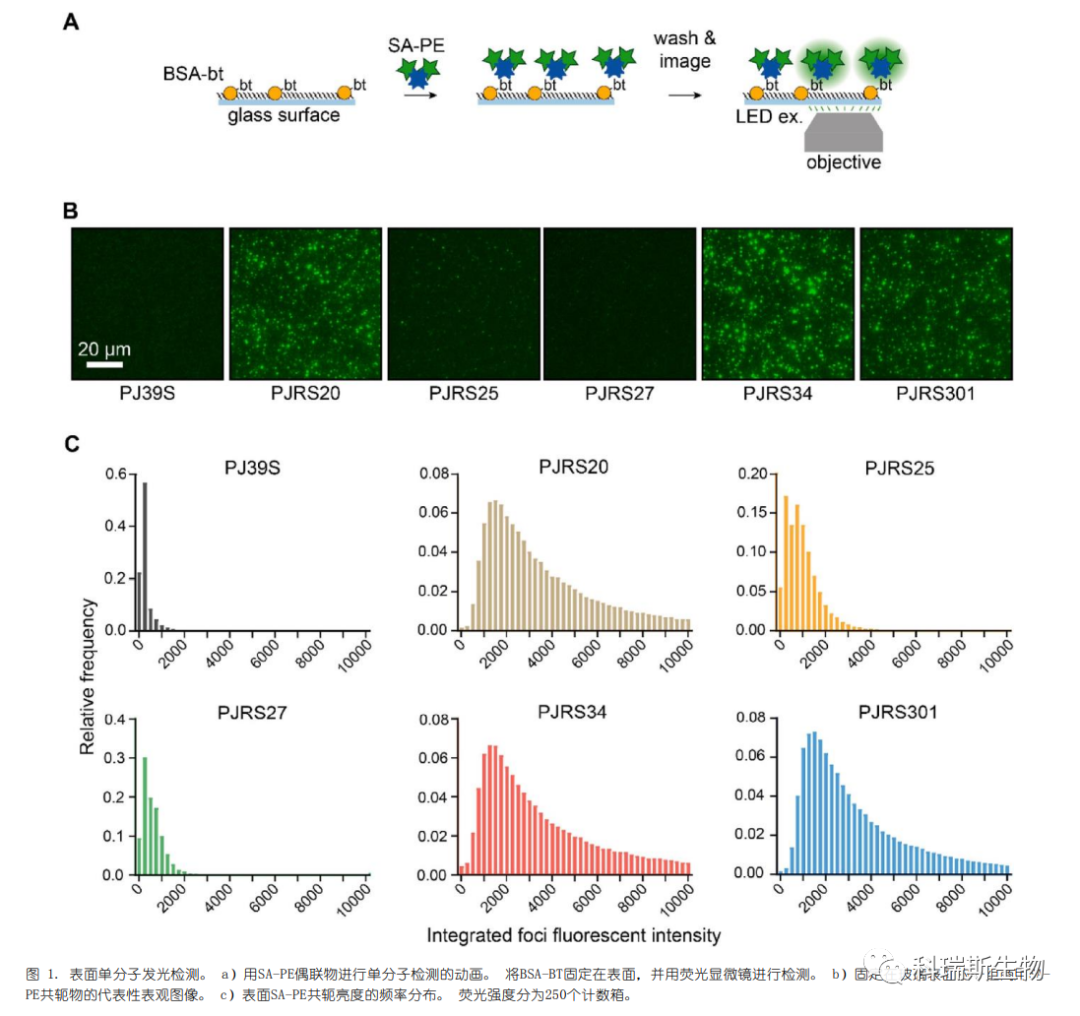
The authors tested whether SA-PE conjugates could function in a "TIRF-like" surface detection assay, where fluorescent molecules would localize to the glass interface and be imaged. TIRF microscopy reduces background intensity by illuminating only a thin layer of the sample near the optical interface. In contrast, epifluorescent microscopy collects light from the entire sample, obscuring individual fluorophores. Therefore, when using epifluorescent microscopy, a bright fluorescent conjugate is required to enhance the signal above the background.
First, biotinylated bovine serum albumin (BSA-bt) was deposited on the glass surface before passivation. Then, SA-PE conjugates were added separately to detect the immobilized BSA (Figure 1a). Individual SA-PE conjugates could be resolved on the glass surface (Figure 1b). It was concluded that these SA-PE conjugates successfully provided distinguishable single-molecule signals.
To test whether the known quantity of an analyte on the glass surface could be enumerated, the authors developed a single-molecule counting assay (Figure 2a). SA-PE (PJRS27), which exhibits lower background surface binding despite not being as bright as other conjugates (Figures S2a and b), was selected. Streptavidin was incubated in 96-well plates to adhere non-specifically to the surface. Biotinylated mouse monoclonal antibodies (mAb-bt) were used as the analyte. Measurements of mAb-bt were performed at an initial concentration of 500 pM, followed by two-fold serial dilutions down to 7.8 pM (Figure 2b). A linear correlation was observed between the number of foci and the concentration of the mAb-bt analyte (Figure 2c). The limited non-specific background binding enabled the SA-PE conjugates to achieve a sensitivity of less than 20 pM, demonstrating that SA-PE can be used for the detection and quantification of surface-bound analytes.
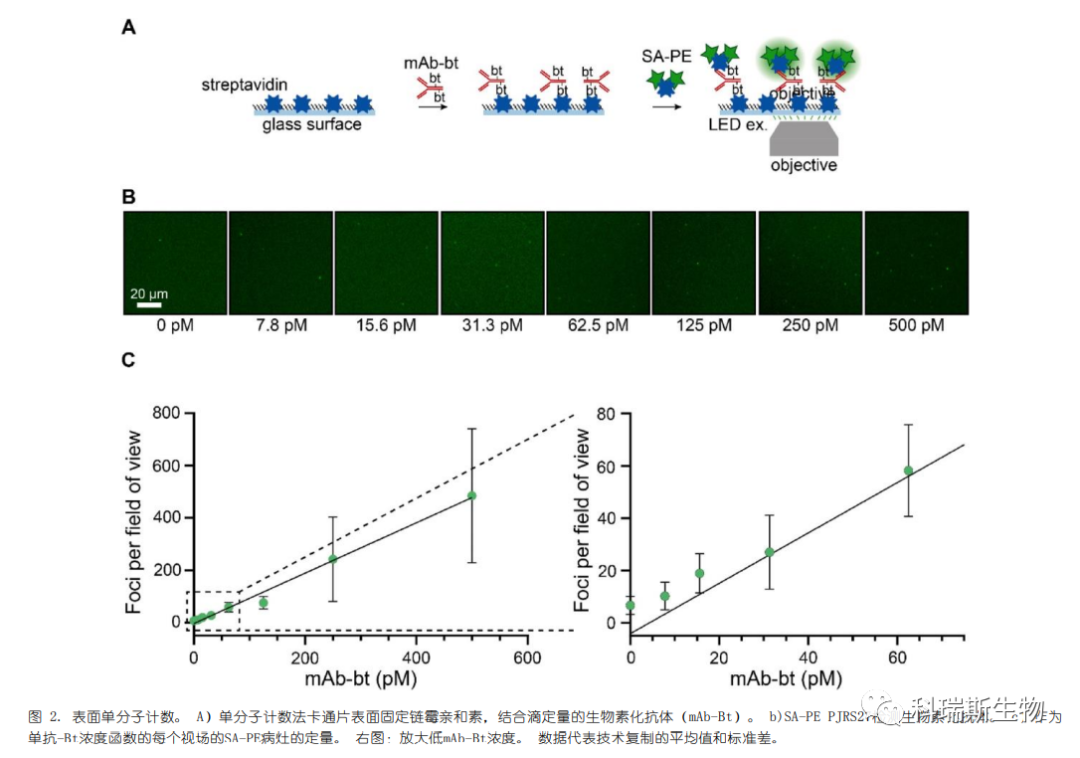
2.Single-Molecule Diagnostics
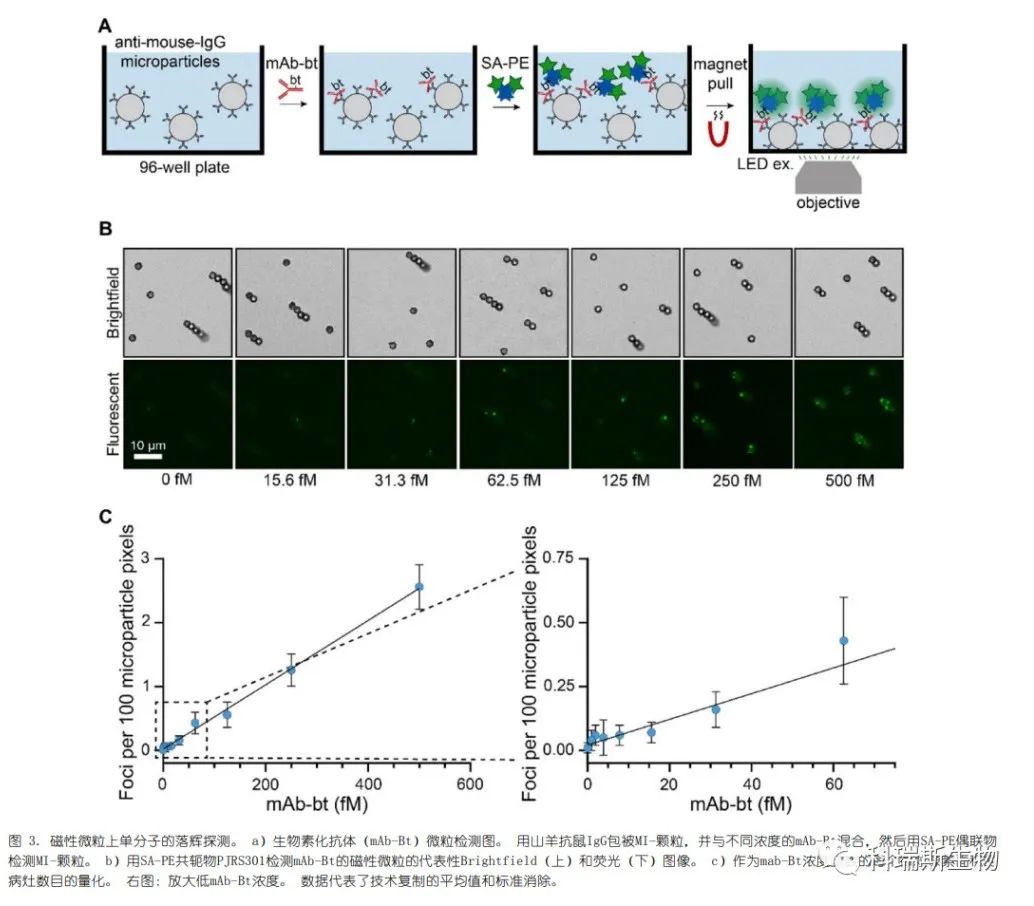
First, the fluorescent signal was tested in a model assay for detecting the same biotinylated antibody (mAb-Bt), as shown in Figure 2 (Figure 3a). Magnetic microparticles were coated with goat anti-mouse antibodies and then incubated with a series of femtomolar concentrations of mAb-Bt. The microspheres were washed, incubated with SA-PE conjugates, washed again, and transferred to a glass baseplate for imaging. We clearly observed individual SA-PE bindings on the microparticles (Figure 3b). Similarly, the three bright SA-PE conjugates—PJRS20, PJRS34, and PJRS301—generated high signals (Figure 3c, Figure S3). The moderately bright conjugates, PJRS25 and PJRS27, were both visible on the microparticles, while the dim conjugate PJ39S was barely distinguishable (Figure S3). Four out of the six SA-PE conjugates showed a linear correlation with the recognized targets and mAb-Bt concentrations; both PJ39S and PJRS27 were too dim for accurate quantification. The differences among the three bright SA-PE conjugates did not affect the detection sensitivity of the method, with the lower limit of sensitivity for all methods being approximately 15 fM (Figure 3c, Figure S3). Therefore, we demonstrated that SA-PE conjugates can be used for digital-based detection in model diagnostic assays.
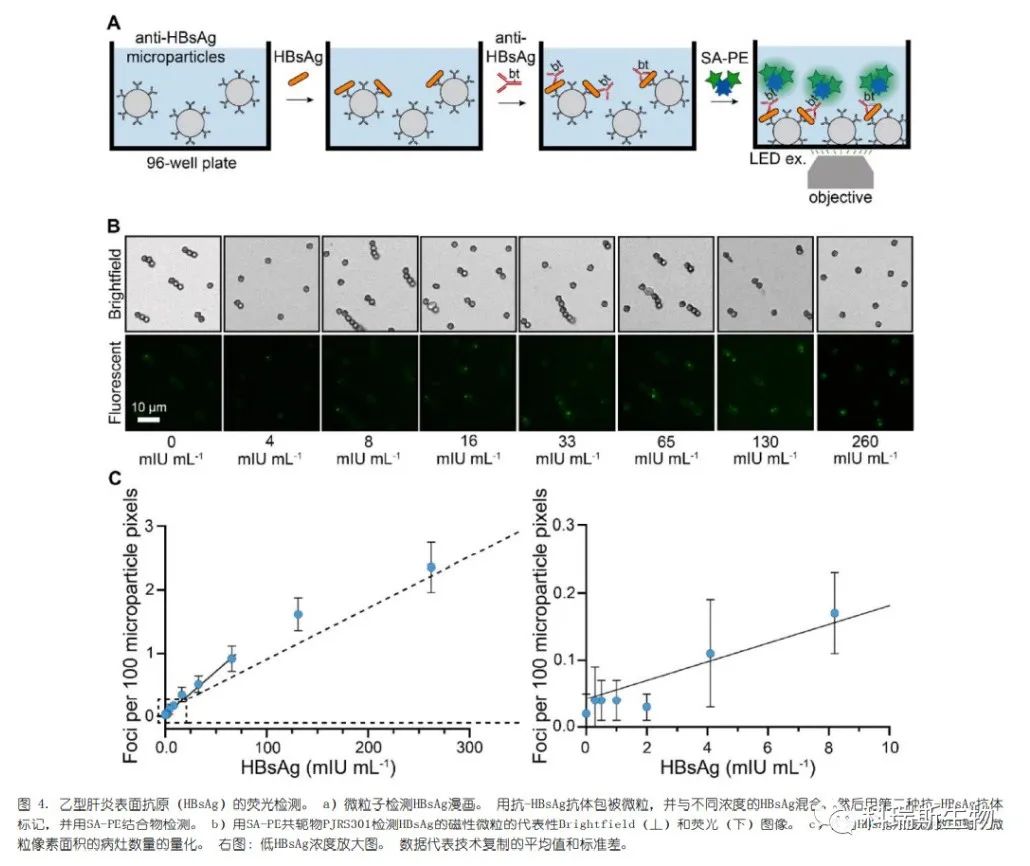
The three bright SA-PE conjugates selected in the preliminary screening were used to test their non-specific binding to microparticles. PJRS20 exhibited significant non-specific binding, while PJRS301 showed better performance and was thus selected for analyte detection (Figure S4). Once again, we could clearly resolve individual foci on the microparticles, and these microparticles displayed a dose response to the dilution of hepatitis B surface antigen (HBsAg) (Figure 4b). This digital assay showed a lower limit of sensitivity for HBsAg of 4.1 mIU mL?1, representing an approximately 5-fold increase in sensitivity compared to commercial chemiluminescence assays (Figure 4c). Therefore, this demonstrates that commercial SA-PE conjugates can be used for the sensitive detection of clinically relevant analytes.
3.Single-Molecule Cell Surface Imaging
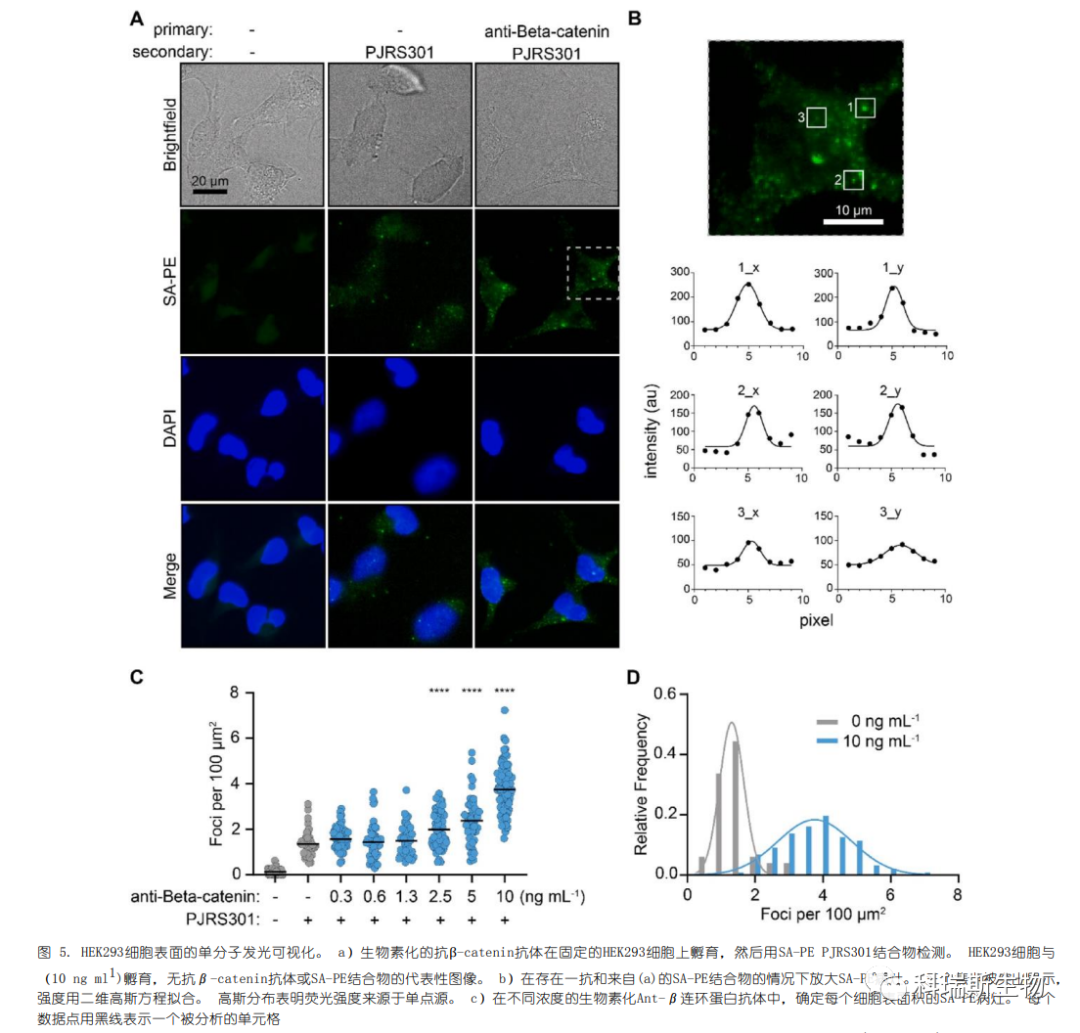
The evolutionarily conserved and widely expressed cell surface protein β-catenin was tested for single-molecule imaging on cultured HEK293 cells. β-catenin is a structural component of adherens junctions and an effector of the Wnt signaling pathway. Immunofluorescence imaging using an anti-rabbit antibody conjugated to Alexa Fluor 594 confirmed the surface expression of β-catenin (Figure S5). Due to the high expression level of β-catenin, we titrated the concentration of the anti-β-catenin primary antibody to be lower than that in standard immunofluorescence protocols. Distinct SA-PE foci on the cell membrane of HEK293 cells were clearly visible (Figure 5A).
Single foci were selected and fitted with a Gaussian function to confirm they were single-point sources (Figure 5B). Control experiments without primary antibodies still showed visible and quantifiable foci, indicating non-specific adsorption of SA-PE on the cell surface. In the presence of 10 ng mL−1 primary antibody and SA-PE, there was an average of 3.8 foci per 100 μm2, compared to 1.4 foci per 100 μm2 with SA-PE alone (Figure 5C). On fixed cells without SA-PE, only 0.1 foci per 100 μm2 were detected. When the primary antibody concentration exceeded 10 ng/mL, it caused fluorescence overlap of foci and a reduction in distinguishable foci.
Therefore, we examined the distribution of cells with 0 or 10 ng/mL primary antibody and fitted the spread with a Gaussian function (Figure 5D). There was some overlap between the two populations in regions with 2–3 foci per 100 μm2. Consequently, individual cells could not be accurately assigned to either condition, but population-level analysis did reveal distinct distributions. Thus, single-molecule imaging with SA-PE is most suitable for semi-quantitative or binary analysis of the two populations.
三、Conclusion
To date, commercial SA-PE has been used for average measurements, such as flow cytometry. This article demonstrates that SA-PE can be adapted for digital detection applications. It provides researchers with a more easily adaptable framework for single-molecule counting. Based on the biotin-streptavidin interaction, it offers a versatile “universal” platform for detecting a wide range of analytes. This technology can be further expanded by using other phycobiliproteins conjugated with detection antibodies (e.g., anti-FLAG antibodies).
References:Epifluorescent single-molecule counting with Streptavidin-Phycoerythrin conjugates.
|